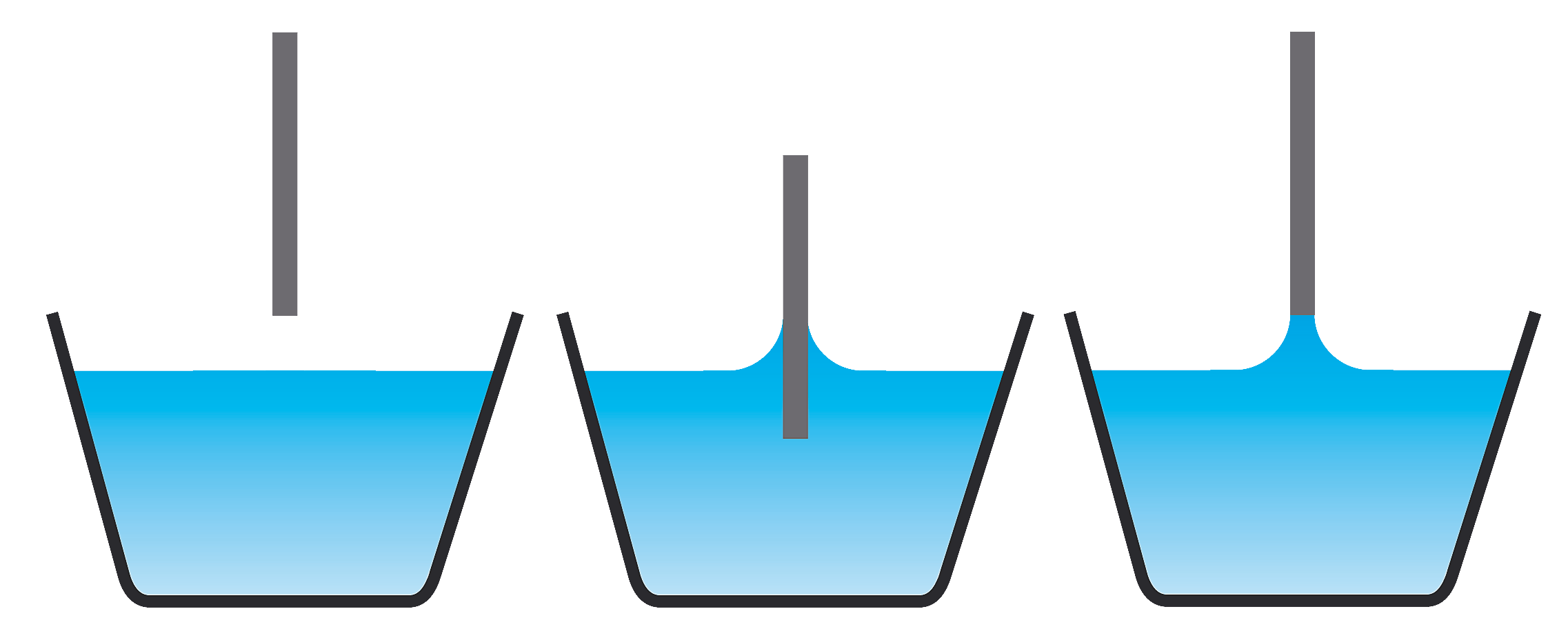
What Has A Higher Surface Tension At 298K . surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Water has a surface tension of. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
from www.kibron.com
89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Water has a surface tension of. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension may be expressed, therefore, in units of energy per unit area (square metres).
Surface Tension What is surface tension Kibron
What Has A Higher Surface Tension At 298K surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. surface tension may be expressed, therefore, in units of energy per unit area (square metres). 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Has A Higher Surface Tension At 298K water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Water has a surface tension of. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. 89 rows this table highlights the viscosity and surface tension of a variety of solvents. What Has A Higher Surface Tension At 298K.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and What Has A Higher Surface Tension At 298K Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Water has a surface tension of.. What Has A Higher Surface Tension At 298K.
From www.researchgate.net
Variation of surface tension (γ) with log[S] at 298 K of the AOT−IBF What Has A Higher Surface Tension At 298K surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components. What Has A Higher Surface Tension At 298K.
From www.numerade.com
SOLVED Researchers measured the surface tension of various solutions What Has A Higher Surface Tension At 298K water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the tendency. What Has A Higher Surface Tension At 298K.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) What Has A Higher Surface Tension At 298K 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Water has a surface tension of. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components. What Has A Higher Surface Tension At 298K.
From stock.adobe.com
surface tension wetting surfactant water hydrophilic hydrophobic What Has A Higher Surface Tension At 298K water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension may be expressed, therefore, in units of energy per unit area (square metres). 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. Surface tension is the tendency. What Has A Higher Surface Tension At 298K.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru What Has A Higher Surface Tension At 298K 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Water has a surface tension. What Has A Higher Surface Tension At 298K.
From mavink.com
What Is Surface Tension What Has A Higher Surface Tension At 298K surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. 89 rows this table. What Has A Higher Surface Tension At 298K.
From www.researchgate.net
The surface tension isotherms of different block polyethers at 298 K What Has A Higher Surface Tension At 298K Water has a surface tension of. surface tension may be expressed, therefore, in units of energy per unit area (square metres). water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible.. What Has A Higher Surface Tension At 298K.
From dxorquocc.blob.core.windows.net
What Does High Surface Tension Mean at Freda Bourne blog What Has A Higher Surface Tension At 298K Water has a surface tension of. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. . What Has A Higher Surface Tension At 298K.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Has A Higher Surface Tension At 298K surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension. What Has A Higher Surface Tension At 298K.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Has A Higher Surface Tension At 298K 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension. What Has A Higher Surface Tension At 298K.
From www.learnatnoon.com
What is Surface Tension in Physics? What Has A Higher Surface Tension At 298K Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. What Has A Higher Surface Tension At 298K.
From dxorquocc.blob.core.windows.net
What Does High Surface Tension Mean at Freda Bourne blog What Has A Higher Surface Tension At 298K Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension may be expressed, therefore, in units of energy per unit area (square metres). 89 rows this table. What Has A Higher Surface Tension At 298K.
From www.vrogue.co
Surface Tension Definition Units Epic Examples Effect vrogue.co What Has A Higher Surface Tension At 298K surface tension may be expressed, therefore, in units of energy per unit area (square metres). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. water, for example,. What Has A Higher Surface Tension At 298K.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Has A Higher Surface Tension At 298K Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Water has a surface tension of. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. surface tension is the energy, or work, required to increase the surface area of a liquid. What Has A Higher Surface Tension At 298K.
From www.vrogue.co
Surface Tension Definition Examples And Unit vrogue.co What Has A Higher Surface Tension At 298K surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the. What Has A Higher Surface Tension At 298K.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Has A Higher Surface Tension At 298K water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Water has a surface tension of. 89 rows this table highlights the viscosity and surface tension of a variety of solvents and other liquids. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum. What Has A Higher Surface Tension At 298K.